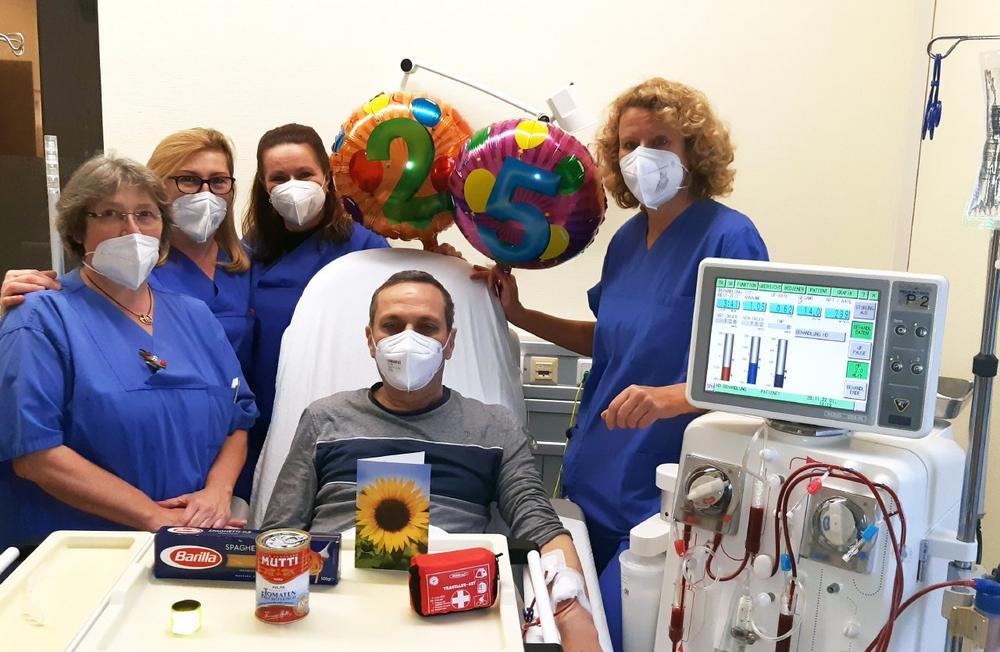
„Das Knappschaftskrankenhaus ist beinahe ein zweites Zuhause für mich. Seit 25 Jahren komme ich hierher und ich habe mich immer wohlgefühlt. Einige Pflegekräfte kenne ich von Anfang an. Sie sind den ganzen Weg mit mir zusammen gegangen“, erzählt Giuseppe Rapisade und freut sich über die Aufmerksamkeiten, die das Dialyseteam anlässlich seines besonderen Jubiläums für ihn […]
continue reading