The spread of digitalization and connection throughout all areas of industry has been accompanied by increasingly safety requirements which systems and components must fulfill. At SPS 2024, held in Nuremberg from November 12–14, 2024, TÜV SÜD will provide information on its testing and certification services for functional safety and industrial IT security in a wide […]
continue readingTaking an integrated approach to functional safety and industrial IT security
The spread of digitalization and connection throughout all areas of industry has been accompanied by increasingly safety requirements which systems and components must fulfill. At SPS 2024, held in Nuremberg from November 12–14, 2024, TÜV SÜD will provide information on its testing and certification services for functional safety and industrial IT security in a wide […]
continue reading
Paula Pias Peleteiro verstärkt Geschäftsführung der TÜV SÜD Industrie Service GmbH
Paula Pias Peleteiro wurde in die Geschäftsführung der TÜV SÜD Industrie Service GmbH berufen. Sie übernimmt die Verantwortung für die Geschäftsfelder Anlagensicherheit, Energie & Systeme sowie Umwelttechnik. Mit bedarfsgerechten Ingenieur- und Prüfleistungen sorgt TÜV SÜD Industrie Service für Sicherheit, Wirtschaftlichkeit und Nachhaltigkeit von Anlagen, Infrastruktureinrichtungen und Gebäuden. Vor ihrem Wechsel zu TÜV SÜD war Paula […]
continue reading
Paula Pias Peleteiro verstärkt Geschäftsführung der TÜV SÜD Industrie Service GmbH
Paula Pias Peleteiro wurde in die Geschäftsführung der TÜV SÜD Industrie Service GmbH berufen. Sie übernimmt die Verantwortung für die Geschäftsfelder Anlagensicherheit, Energie & Systeme sowie Umwelttechnik. Mit bedarfsgerechten Ingenieur- und Prüfleistungen sorgt TÜV SÜD Industrie Service für Sicherheit, Wirtschaftlichkeit und Nachhaltigkeit von Anlagen, Infrastruktureinrichtungen und Gebäuden. Vor ihrem Wechsel zu TÜV SÜD war Paula […]
continue reading
Sabine Nitzsche appointed as new CFO of TÜV SÜD AG
Sabine Nitzsche has been appointed by the Supervisory Board of TÜV SÜD AG as the new Chief Financial Officer and Member of the Board of Management with effect from 1 March 2025. She succeeds Prof. Matthias J. Rapp, who left the company on 30 September 2024. TÜV SÜD is one of the leading international providers of […]
continue reading
Sabine Nitzsche appointed as new CFO of TÜV SÜD AG
Sabine Nitzsche has been appointed by the Supervisory Board of TÜV SÜD AG as the new Chief Financial Officer and Member of the Board of Management with effect from 1 March 2025. She succeeds Prof. Matthias J. Rapp, who left the company on 30 September 2024. TÜV SÜD is one of the leading international providers of […]
continue reading
Da stimmt die Chemie: Wasserstoff kennt keine Grenzen
Mit der Entwicklung des Energieträgers Wasserstoff in der chemi-schen Industrie und in benachbarten Branchen befasst sich das TÜV SÜD H2-Forum RheinBelgien, das am 13. November 2024 als Hybrid-Veranstaltung stattfindet. Das Spektrum der Vorträge reicht von Infrastrukturprojekten über Anwendungsbeispiele bis zu Prüf- und Zertifiziermöglichkeiten. Der Einsatz von Wasserstoff soll nach dem Willen der Bundesregierung in allen […]
continue reading
Da stimmt die Chemie: Wasserstoff kennt keine Grenzen
Mit der Entwicklung des Energieträgers Wasserstoff in der chemi-schen Industrie und in benachbarten Branchen befasst sich das TÜV SÜD H2-Forum RheinBelgien, das am 13. November 2024 als Hybrid-Veranstaltung stattfindet. Das Spektrum der Vorträge reicht von Infrastrukturprojekten über Anwendungsbeispiele bis zu Prüf- und Zertifiziermöglichkeiten. Der Einsatz von Wasserstoff soll nach dem Willen der Bundesregierung in allen […]
continue reading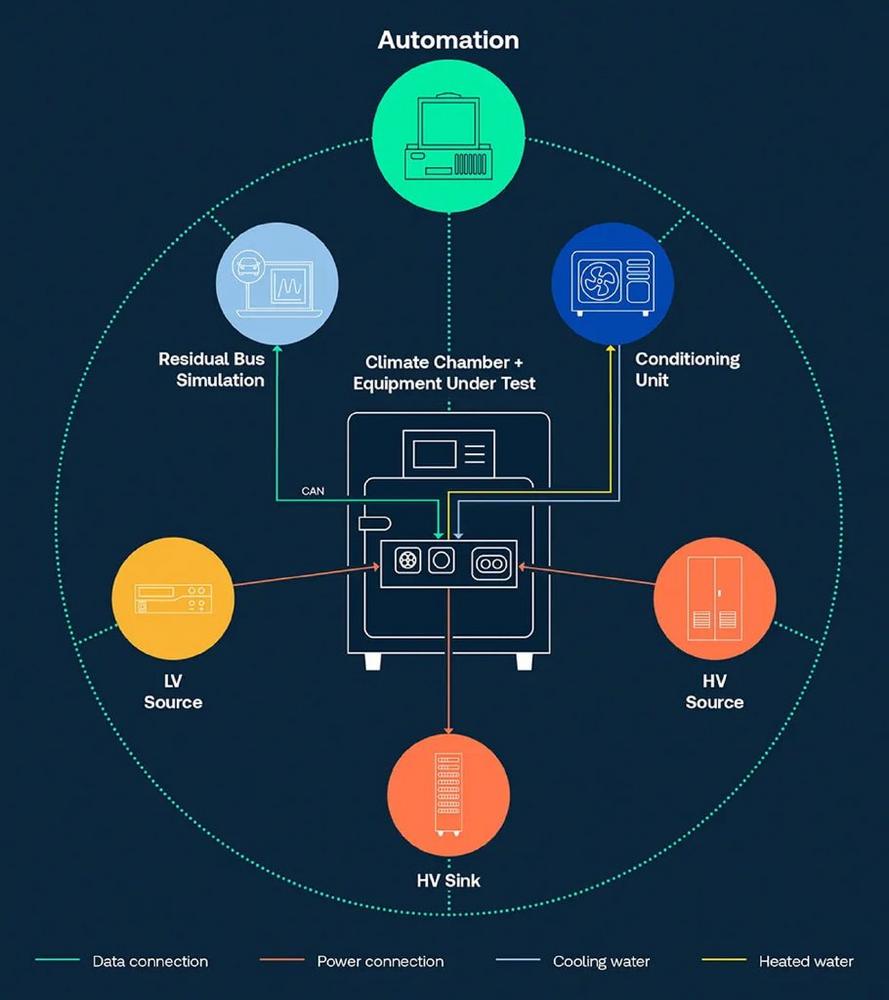
TÜV SÜD drives innovations in HV component testing for electric vehicles
In the face of growing demand for electric vehicles (EVs) and the associated high-voltage technologies, the ability to assure the safety and reliability of their key components is vital. TÜV SÜD is setting new standards in testing of high-voltage components for electric vehicles (HV testing). This testing is crucial in ensuring the safety, performance, and […]
continue reading
TÜV SÜD drives innovations in HV component testing for electric vehicles
In the face of growing demand for electric vehicles (EVs) and the associated high-voltage technologies, the ability to assure the safety and reliability of their key components is vital. TÜV SÜD is setting new standards in testing of high-voltage components for electric vehicles (HV testing). This testing is crucial in ensuring the safety, performance, and […]
continue reading