TÜV SÜD hat zwei Hydrogen Power Cubes (HPC) des chinesischen Herstellers COSBER Technology zertifiziert. HPC sind integrierte Systeme zur autarken Energieversorgung von Gebäuden. Insgesamt drei Zertifikate bestätigen die allgemeine Sicherheit der Geräte und die Konformität mit den europäischen Druckgeräte- und EMV-Richtlinien. COSBER Technology ist einer der weltweit führenden Hersteller von Fahrzeugprüftechnik. Zudem entwickelt das Unternehmen […]
continue readingTÜV SÜD zertifiziert Hydrogen Power Cubes (HPC) von COSBER Technology
TÜV SÜD hat zwei Hydrogen Power Cubes (HPC) des chinesischen Herstellers COSBER Technology zertifiziert. HPC sind integrierte Systeme zur autarken Energieversorgung von Gebäuden. Insgesamt drei Zertifikate bestätigen die allgemeine Sicherheit der Geräte und die Konformität mit den europäischen Druckgeräte- und EMV-Richtlinien. COSBER Technology ist einer der weltweit führenden Hersteller von Fahrzeugprüftechnik. Zudem entwickelt das Unternehmen […]
continue readingKostenloser Online-Infoabend zur MPU
Zu viele Punkte in Flensburg, Alkohol oder Drogen am Steuer – es gibt verschiedene Gründe, weshalb eine Medizinisch-Psychologische Untersuchung (MPU) angeordnet werden kann. Andrea Häußler, Verkehrsexpertin und Mitglied der Geschäftsleitung der TÜV SÜD Life Service GmbH, weiß, wie wichtig eine frühzeitige Vorbereitung ist. „Steht eine MPU an, wissen viele Betroffene erst einmal nicht, was auf […]
continue readingKostenloser Online-Infoabend zur MPU
Zu viele Punkte in Flensburg, Alkohol oder Drogen am Steuer – es gibt verschiedene Gründe, weshalb eine Medizinisch-Psychologische Untersuchung (MPU) angeordnet werden kann. Andrea Häußler, Verkehrsexpertin und Mitglied der Geschäftsleitung der TÜV SÜD Life Service GmbH, weiß, wie wichtig eine frühzeitige Vorbereitung ist. „Steht eine MPU an, wissen viele Betroffene erst einmal nicht, was auf […]
continue reading
Funktionierende Gebäudeautomation entscheidend für Energieeffizienz und Nutzerkomfort
Das neue Gebäudeenergiegesetz hat die Anforderungen an die Gebäudeautomation weiter verschärft. Der Fokus liegt auf der Verbesserung der Energieeffizienz. Darüber hinaus ist eine richtig geplante und gut funktionierende Gebäudeautomation ein wesentlicher Faktor für den Nutzerkomfort und den Werterhalt eines Gebäudes. TÜV SÜD informiert Bauherren mit dem Whitepaper „Gebäudeautomation richtig planen und betreiben“ über Schlüsselthemen für […]
continue reading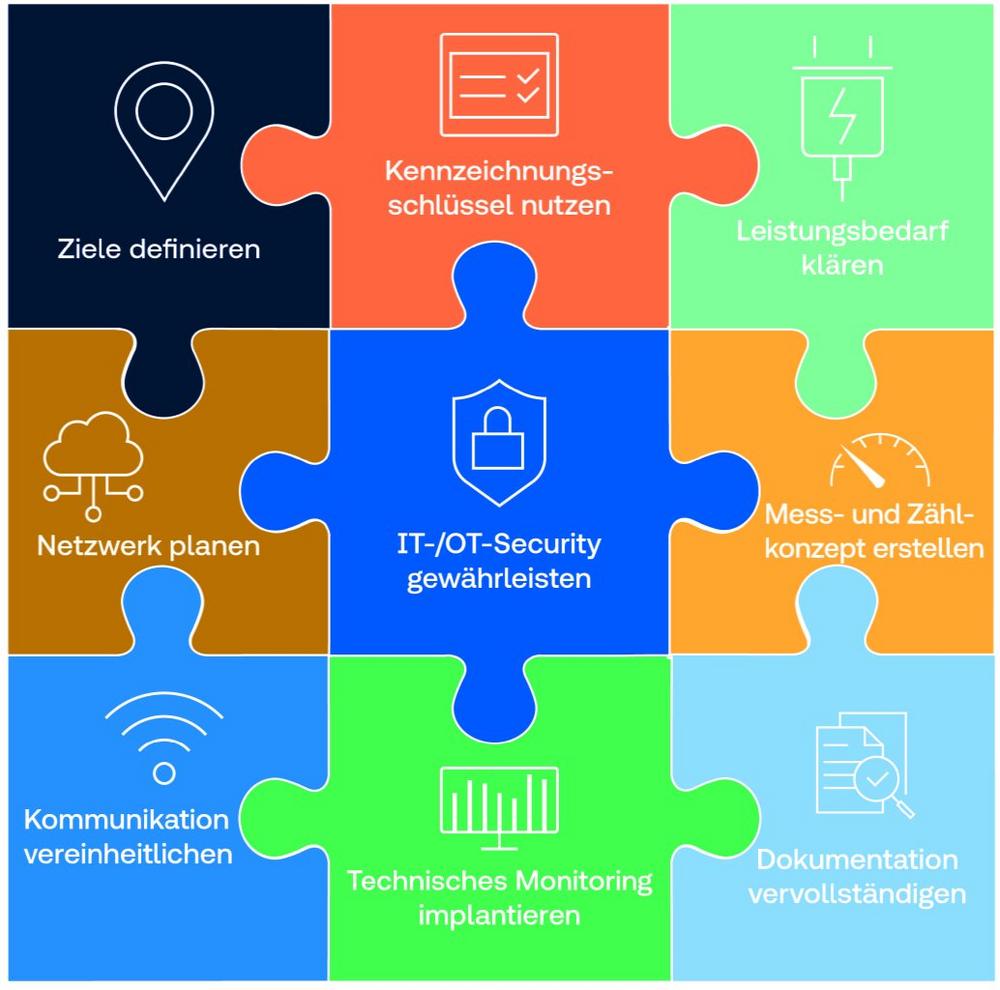
Funktionierende Gebäudeautomation entscheidend für Energieeffizienz und Nutzerkomfort
Das neue Gebäudeenergiegesetz hat die Anforderungen an die Gebäudeautomation weiter verschärft. Der Fokus liegt auf der Verbesserung der Energieeffizienz. Darüber hinaus ist eine richtig geplante und gut funktionierende Gebäudeautomation ein wesentlicher Faktor für den Nutzerkomfort und den Werterhalt eines Gebäudes. TÜV SÜD informiert Bauherren mit dem Whitepaper „Gebäudeautomation richtig planen und betreiben“ über Schlüsselthemen für […]
continue reading
Neuer Bisphenol A-Grenzwert für Trinkwasser
Trinkwasser ist in Deutschland ein hoch qualitatives und sehr streng kontrolliertes Lebensmittel. Nun legt eine, im vorigen Jahr neu gefasste, Trinkwasserverordnung zusätzlich einen Grenzwert für die verbreitete Substanz Bisphenol A fest. Sie wird bei der Herstellung von Kunststoffen und -harzen verwendet. TÜV SÜD informiert, welche neuen Regelungen jetzt für Bisphenol A gelten und warum auch […]
continue reading
Neuer Bisphenol A-Grenzwert für Trinkwasser
Trinkwasser ist in Deutschland ein hoch qualitatives und sehr streng kontrolliertes Lebensmittel. Nun legt eine, im vorigen Jahr neu gefasste, Trinkwasserverordnung zusätzlich einen Grenzwert für die verbreitete Substanz Bisphenol A fest. Sie wird bei der Herstellung von Kunststoffen und -harzen verwendet. TÜV SÜD informiert, welche neuen Regelungen jetzt für Bisphenol A gelten und warum auch […]
continue reading
TÜV SÜD-Tipps für den optimalen Durchblick
Der Frühjahrsputz steht an, doch Fensterputzen gehört dabei für viele nicht unbedingt zur Lieblingsbeschäftigung. Der Grund sind lästige Schlieren auf den Scheiben und schmutziges Wasser, das auf den Boden tropft. Doch Akku-Fenstersauger schaffen Abhilfe. Dank ihrer starken Saugleistung reinigen die flexiblen Geräte glatte Oberflächen wie Glas, Spiegel oder Fliesen streifenfrei, fangen herabfließendes Schmutzwasser sofort auf […]
continue reading
TÜV SÜD-Tipps für den optimalen Durchblick
Der Frühjahrsputz steht an, doch Fensterputzen gehört dabei für viele nicht unbedingt zur Lieblingsbeschäftigung. Der Grund sind lästige Schlieren auf den Scheiben und schmutziges Wasser, das auf den Boden tropft. Doch Akku-Fenstersauger schaffen Abhilfe. Dank ihrer starken Saugleistung reinigen die flexiblen Geräte glatte Oberflächen wie Glas, Spiegel oder Fliesen streifenfrei, fangen herabfließendes Schmutzwasser sofort auf […]
continue reading